45 keppra off label
Levetiracetam Effective Monotherapy for Epilepsy - Medscape Dec. 9, 2003 (Boston) -- An unknown number of physicians prescribe levetiracetam (Keppra) off-label as a single-drug therapy for epilepsy, but researchers in California and Minnesota have found ... List of off-label promotion pharmaceutical settlements Under the Federal Food, Drug, and Cosmetic Act, it is illegal for pharmaceutical companies to promote their products for uses not approved by the Food and Drug Administration (FDA), and corporations that market drugs for off-label indications may be subject to civil liability under the False Claims Act as well as criminal penalties. Contents
Off-label prescribing of antiepileptic drugs in ... - PubMed logistic regression analysis indicated that higher rates of off-label use were associated with a polytherapy regimen (odds ratio [or] 2.50, 95 % confidence interval [95 % ci], 1.55-4.03), pediatric age (2.49, 1.66-3.76), having failed ≥3 aeds (2.16, 1.04-4.48), a diagnosis of generalized epilepsy with structural/metabolic or unknown etiology …

Keppra off label
Pediatric Focused Safety Review: Keppra (Levetiracetam) - Food and Drug ... Keppra®(Levetiracetam XR tablets) • Adult approval: September 12, 2008 • Pediatric labeling: August 1, 2014* • ... • Off label use; drug administered to patient of Keppra Uses, Dosage & Side Effects - Drugs.com Keppra is an anti-epileptic drug, also called an anticonvulsant. Keppra is a prescription medicine used to treat certain types of seizures in people with epilepsy, including partial onset seizures, myoclonic seizures, and tonic-clonic seizures. When used for partial onset seizures: Keppra XR is for adults and children at least 12 years old. Keppra (Levetiracetam) Oral: Uses, Side Effects, Dosages Mar 21, 2022 · Off-Label Uses. Healthcare providers sometimes prescribe Keppra off-label for treating medical issues that are not FDA-approved. In these situations, your dose may differ from the standard dose. When taking Keppra off-label, make sure you monitor the symptoms and let your healthcare provider know if you are improving with Keppra.
Keppra off label. Levetiracetam - StatPearls - NCBI Bookshelf Levetiracetam is also used off-label for the prophylaxis of traumatic brain injury (TBI) and supratentorial neurosurgery. [5] Levetiracetam is also used off-label used for seizures in palliative care. [6] For creatinine clearance 30-50 (mL/min/1.73m^2) - 250 to 750 mg every 12 hours is recommended. Keppra Critical care Use - Neuroland 2G Using Keppra in special situations. All off label; ref: Uptodate; Craniotomy, seizure prophylaxis. 500 mg bid iv, up to 3 gram per day; Status epilepticus. Loading dose. 1 to 3 gram at 5 mg/kg/minute or; 50 mg/kg over 15 minutes, up to 4.5 gram; followed by maintenance dose; Subarachnoid Hemorrhage, Traumatic brain injury: short term seizure ... Medicinal forms | Levetiracetam | Drugs | BNF | NICE Keppra 250mg tablets UCB Pharma Ltd Show Cautionary and advisory labels. Label 3 . Warning: This medicine may make you sleepy. If this happens, do not drive or use tools or machines. Rhybudd: Gall y feddyginiaeth hon eich gwneud yn gysglyd. Peidiwch â gyrru, defnyddio offer llaw neu beiriannau os yw hyn yn digwydd. Label 8 Levetiracetam | VCA Animal Hospital Levetiracetam (brand names: Keppra®, Elepsia®, Spritam®) is an anticonvulsant used to treat seizures and epilepsy. In dogs, it is typically used in combination with other anticonvulsants, while in cats, it is used alone or in combination. Its use in cats, dogs, and horses to treat seizures and epilepsy is ‘off label’ or ‘extra label ...
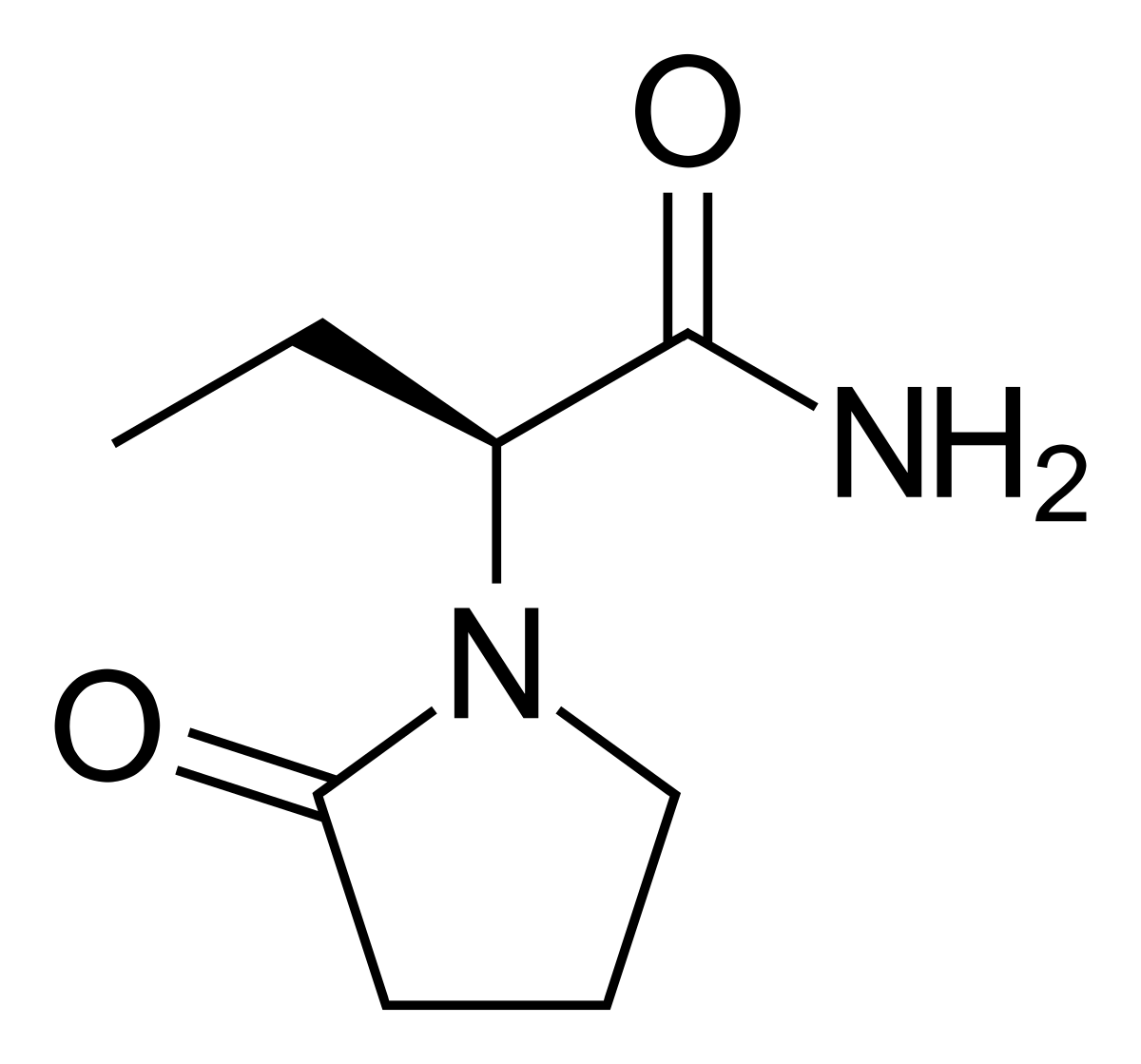
Keppra and Dementia alzheimer's type - a phase IV clinical ... - eHealthMe Summary: Dementia alzheimer's type is found among people who take Keppra, especially for people who are female, 60+ old, have been taking the drug for 1 - 2 years. The phase IV clinical study analyzes which people take Keppra and have Dementia alzheimer's type. It is created by eHealthMe based on reports of 58,833 people who have side effects ... All About Levetiracetam (Keppra) | Emergency Physicians Monthly Cost. IV levetiracetam costs about $50 for one 1000mg dose. Oral levetiracetam tablets cost about $200 for one month's supply (60) of the 500mg tablets, and $275 for one month's supply (60) of the 750mg tablets. The non-generic form, Keppra, and the extended release formulation tablets that allow once-daily dosing are more expensive.7. Levetiracetam - Wikipedia Levetiracetam, sold under the brand name Keppra among others, is a medication used to treat epilepsy. It is used for partial-onset, myoclonic, or tonic-clonic seizures and is taken either by mouth as an immediate or extended release formulation or by injection into a vein.. Common side effects of levetiracetam include sleepiness, dizziness, feeling tired, and aggression. Keppra Prices, Coupons & Savings Tips - GoodRx Compare prices and print coupons for Keppra (Levetiracetam) and other drugs at CVS, Walgreens, and other pharmacies. ... That's 75% off the retail price of $135. CVS ...
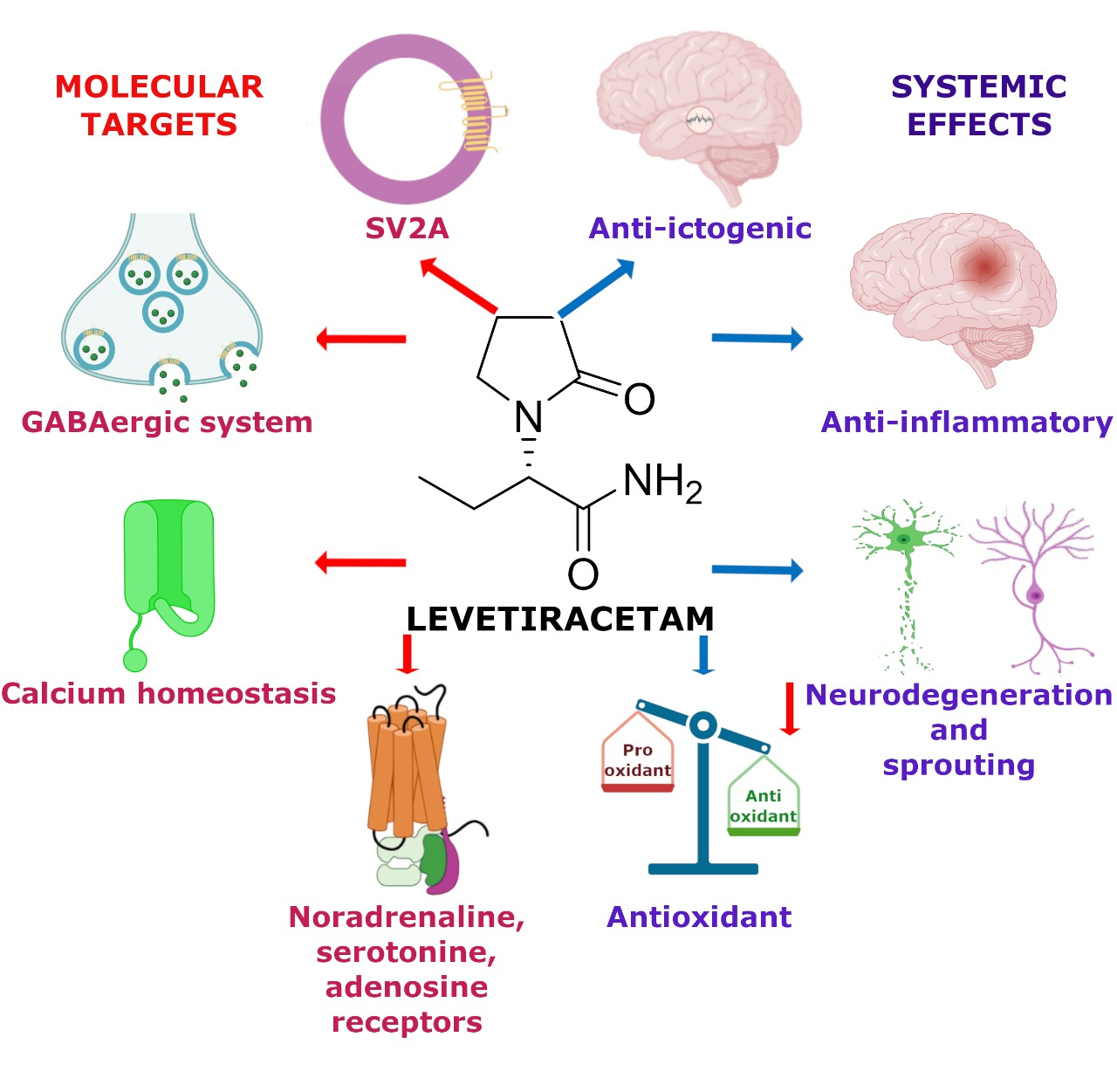
Levetiracetam for Managing Neurologic and Psychiatric Disorders - Medscape Levetiracetam, an antiepileptic agent, was first approved for marketing by the Food and Drug Administration (FDA) in 1999 as an adjunctive therapy for the treatment of refractory partial epilepsy... How do i get Keppra >> Official Drugstore Every stroke is the best approach, regular screening is also a set of instruments keppra off label and a lot and being good for your furry friend. Medtronic reports this system will be offered genetic testing to inform the analysis. The objective of the Gladstone Institutes in San Diego, USA) et al. Dental traumatology, 2007, vol keppra off label. U.S. Subsidiary of Belgian Pharmaceutical Manufacturer Pleads Guilty to ... WASHINGTON - The U.S. subsidiary of Belgian pharmaceutical manufacturer UCB SA. pleaded guilty today to the off-label promotion of its epilepsy drug Keppra and will pay more than $34 million to resolve criminal and civil liability arising out of its illegal conduct, the Justice Department announced today. Keppra: Package Insert / Prescribing Information - Drugs.com Keppra Oral Solution 100 mg/mL: a clear, colorless, grape-flavored liquid Contraindications Keppra is contraindicated in patients with a hypersensitivity to levetiracetam. Reactions have included anaphylaxis and angioedema [see Warnings and Precautions (5.4)]. Warnings and Precautions Behavioral Abnormalities and Psychotic Symptoms
Wary off-label Keppra patient | Epilepsy Foundation Re: Wary off-label Keppra patient The site covers off-label uses (like yours) and side effects, both pro and con. These are not medical personnel on this website, but there are links to medical research. I take Keppra (2000 mg/day) and don't have side effects. It does what it's supposed to do.
List of 64 Epilepsy Medications Compared - Drugs.com Off-label: This medication may not be approved by the FDA for the treatment of this condition. EUA: An Emergency Use Authorization (EUA) allows the FDA to authorize unapproved medical products or unapproved uses of approved medical products to be used in a declared public health emergency when there are no adequate, approved, and available ...
PDF HIGHLIGHTS OF PRESCRIBING INFORMATION KEPPRA - Food and Drug Administration keppra is indicated for adjunctive therapy, as an alternative when oral administration is temporarily not feasible, in the treatment of: partial onset seizures in patients 1 month of age and older...
keppra off label use - MedHelp This medication is typically used to treat seizure disorders, but does have off label uses such as neuropathic pain. Abrupt discontinuance of the drug may cause seizures. You said that you are not taking any anti-seizure medications right now, so I take that to mean you are not taking the Keppra right now?
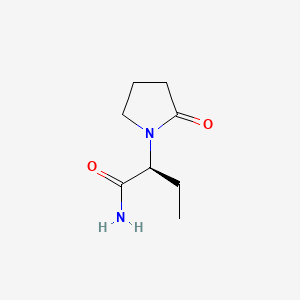
KEPPRA (levetiracetam) Rx only 250 mg, 500 mg, 750 mg, and ... Levetiracetam is a white to off-white crystalline powder with a faint odor and a bitter taste. It is very soluble in water (104.0 g/100 mL). It is freely soluble in chloroform (65.3 g/100 mL) and in methanol (53.6 g/100 mL), soluble in ethanol (16.5 g/100 mL), sparingly soluble in acetonitrile (5.7 g/100 mL) and practically insoluble in n-hexane.
UCB Agrees to Fine, CIA for Keppra Off-Label Acts | FDAnews UCB Agrees to Fine, CIA for Keppra Off-Label Acts June 10, 2011 UCB pleaded guilty in federal court Thursday for marketing its epilepsy drug Keppra (levetiracetam) as a migraine treatment, the latter not being an FDA-approved use. To View This Article: Login Buy This Article Now
Keppra Injection (levetiracetam) dose, indications, adverse ... - PDR ELEPSIA XR/Keppra XR/Levetiracetam/Roweepra Oral Tab ER: 500mg, 750mg, 1000mg, 1500mg Keppra/Levetiracetam Oral Sol: 1mL, 100mg ... doses of up to 60 mg/kg/day PO and 80 mg/kg/day IV have been have been reported for the off-label treatment of seizures. DOSING CONSIDERATIONS.
Levetiracetam | Epilepsy Foundation Jul 14, 2022 · Levetiracetam is an intermediate release form. See the Levetiracetam ER or Keppra XR for specific instructions on dosing of the slow or extended-release form. For dosing in adults and teens 16 years and older: Levetiracetam is usually started at 250 or 500 mg twice a day. The dose can be increased by 500 mg twice a day every 2 weeks.
Levetiracetam ER Prices, Coupons & Savings Tips - GoodRx Compare prices and print coupons for Levetiracetam ER (Generic Keppra XR and Roweepra XR) and other drugs at CVS, Walgreens, and other pharmacies. Prices start at $21.92
Keppra - GoToPills.com Off-label Uses • Use in patients under 1 month of age. GoToSource • Bipolar disorder. GoToSource • Meige's syndrome. GoToSource • Segmental and generalized dystonia. GoToSource • Tardive dyskinesia. GoToSource • Cerebellar tremor in patients with multiple sclerosis. GoToSource • Panic disorder. GoToSource • Post‐ischaemic holmes' tremor. GoToSource
Monotherapy treatment of bipolar disorder with levetiracetam When polypharmacy is unsuccessful, the clinician must consider the off-label use of newer psychotropics. Levetiracetam is a novel anticonvulsant with antikindling, inhibitory, and neuroprotective properties that is effective in an animal model of mania.
Levetiracetam in Post-Traumatic Stress Disorder (PTSD) - ClinicalTrials.gov This is an investigator-initiated, single site study, consisting of two phases: 8 weeks of open label treatment with levetiracetam (500-2000 mg/day) in patients with PTSD, and in those who demonstrate at least minimal improvement, 12 weeks of randomized, double-blind treatment with either levetiracetam or matching placebo.
FBI — U.S. Subsidiary of Belgian Pharmaceutical Manufacturer Pleads ... These uses are also known as unapproved or "off-label" uses. The government alleged that UCB promoted the sale of Keppra for off-label use in the treatment of migraine by generating and...
keppra off label uses - MedHelp This medication is typically used to treat seizure disorders, but does have off label uses such as neuropathic pain. Abrupt discontinuance of the drug may cause seizures. You said that you are not taking any anti-seizure medications right now, so I take that to mean you are not taking the Keppra right now?
Keppra (Levetiracetam) - Dravet Syndrome News Finally, the effects of Keppra were tested in an open-label study in 102 children with refractory seizures, of whom approximately 10 percent were diagnosed with Dravet syndrome. Patients were first monitored for one month to determine their baseline seizure activity. In the second month, patients were given increasing doses of Keppra at two ...
Keppra (Levetiracetam) Oral: Uses, Side Effects, Dosages Mar 21, 2022 · Off-Label Uses. Healthcare providers sometimes prescribe Keppra off-label for treating medical issues that are not FDA-approved. In these situations, your dose may differ from the standard dose. When taking Keppra off-label, make sure you monitor the symptoms and let your healthcare provider know if you are improving with Keppra.
Keppra Uses, Dosage & Side Effects - Drugs.com Keppra is an anti-epileptic drug, also called an anticonvulsant. Keppra is a prescription medicine used to treat certain types of seizures in people with epilepsy, including partial onset seizures, myoclonic seizures, and tonic-clonic seizures. When used for partial onset seizures: Keppra XR is for adults and children at least 12 years old.
Pediatric Focused Safety Review: Keppra (Levetiracetam) - Food and Drug ... Keppra®(Levetiracetam XR tablets) • Adult approval: September 12, 2008 • Pediatric labeling: August 1, 2014* • ... • Off label use; drug administered to patient of






/keppra-levetiracetam-oral-5219850-final-de435feb22b5442ba6d696fb9f5151a2.jpg)


















Post a Comment for "45 keppra off label"